OVER THE PAST 20 YEARS, there has been tremendous growth in the types of technology available to enhance our ability to practice optometry and care for patients. As our profession continues to explode with tech, there are innovations that eyecare practitioners (ECPs) can use to make an impact in practice—especially in specialty areas. By incorporating these innovations, we can help our patients achieve greater success.
Maximizing Soft Lens Fitting With AI
While many clinicians have a preferred trial soft lens, no soft lens will fit every eye.1 In fact, 2 contact lenses that have the same base curve can fit differently, even if they have similar diameters.2 One may expect 2 lenses that have the same labeled parameters to drape over the same cornea in the same manner, but many times that is not the case.
The base curve and diameter are marked on the boxes of standard soft lenses, but there are other parameters that are not labeled—namely, the sagittal depth, modulus, and peripheral curves. Knowing the sagittal depth of a soft lens and matching it to the eye can help ECPs become successful when soft lenses do not fit as expected (Figure 1). Two studies assessed the sagittal depth of soft contact lenses currently available on the market.3 Their results are available in their publications.3,4

While sagittal depth measurement has become more common in the specialty lens world since the introduction of sclerals, clinicians often overlook it when prescribing soft lenses.. One study found that the average total sagittal height of the anterior surface of the eye measured 3,740 microns with a range of 3,140 to 4,040 microns.5 Similarly, another study found an average of 3,735 microns with a range of 3,290 to 4,170 microns.6
Although a patient with an average sagittal depth may achieve a successful contact lens fitting with many lenses on the market, patients with eyes of all shapes and sizes deserve lenses that fit properly. Using the lens sagittal depths found in the previously mentioned studies, combined with artificial intelligence (AI) technology. can substantially maximize your success in fitting patients who have sphere and toric parameters in either daily or reusable lenses.
To accomplish this, create a personalized AI extension. Add all of the graphs and articles through the input feature, or point AI to the appropriate websites. Then point the system to a resource that has a knowledge base of the parameters available for each lens type (+/- power of sphere and cylinder). Next, ask the system to guide you through a series of questions about the lens that you would like. For instance: “What is the patient’s refractive error?” (This query can use the data linked to output the lenses that are available in this power.) “Do you desire a hydrogel or silicone hydrogel?” “Do you desire a frequent replacement or single-use lens?” “What is the patient’s sagittal depth?” AI can be customized to give output based upon a practitioner’s normal practicing model. For example, it could be designed to always suggest lenses made of silicone hydrogel unless unavailable. The patient’s sagittal depth can be determined using a scleral profilometer or an optical coherence tomographer (OCT) that has advanced anterior segment capabilities.
Keeping a computer near your special testing equipment will make entering data into the AI program convenient. In the future, advanced AI electronic health records that have stellar interoperability could become streamlined to feed these examination components into AI, suggesting a new lens for an existing patient or a starting lens that could work well for a new patient.
Tracking Myopia Progression
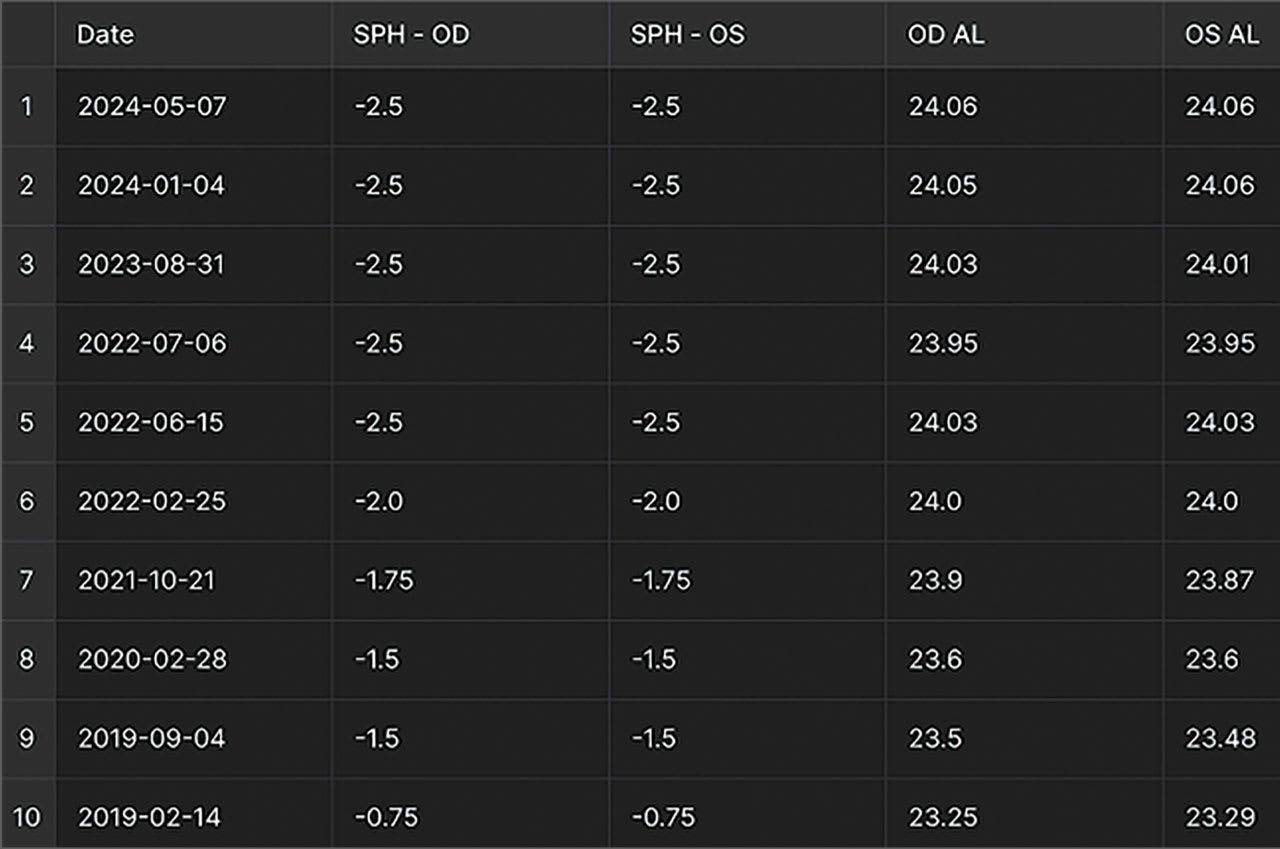
Axial length has become an important parameter to assess. The majority of eyecare offices manage thousands of myopia patients, and the use of axial length enables ECPs to quantify myopia based on more than the refraction of their patients. Axial length is an important factor when considering the risks that a patient may face in developing pathologies.7 Additionally, it allows for a greater understanding of the progression of the disease of myopia, especially in childhood, when myopia progresses the most.8
Recent advances in technology have made instrumentation available that can measure axial length and offer other valuable ocular information, including autorefraction, topography, and assessment of dry eye. Incorporating technology that measures axial length may change the way refractive error and ocular health can be discussed with patients.
One significant challenge ECPs face as myopia management specialists is demonstrating success in slowing myopia progression when communicating with patients and parents. Axial length is an excellent measurement for monitoring myopia control and can be used for all patients, including those wearing orthokeratology (ortho-k) lenses.
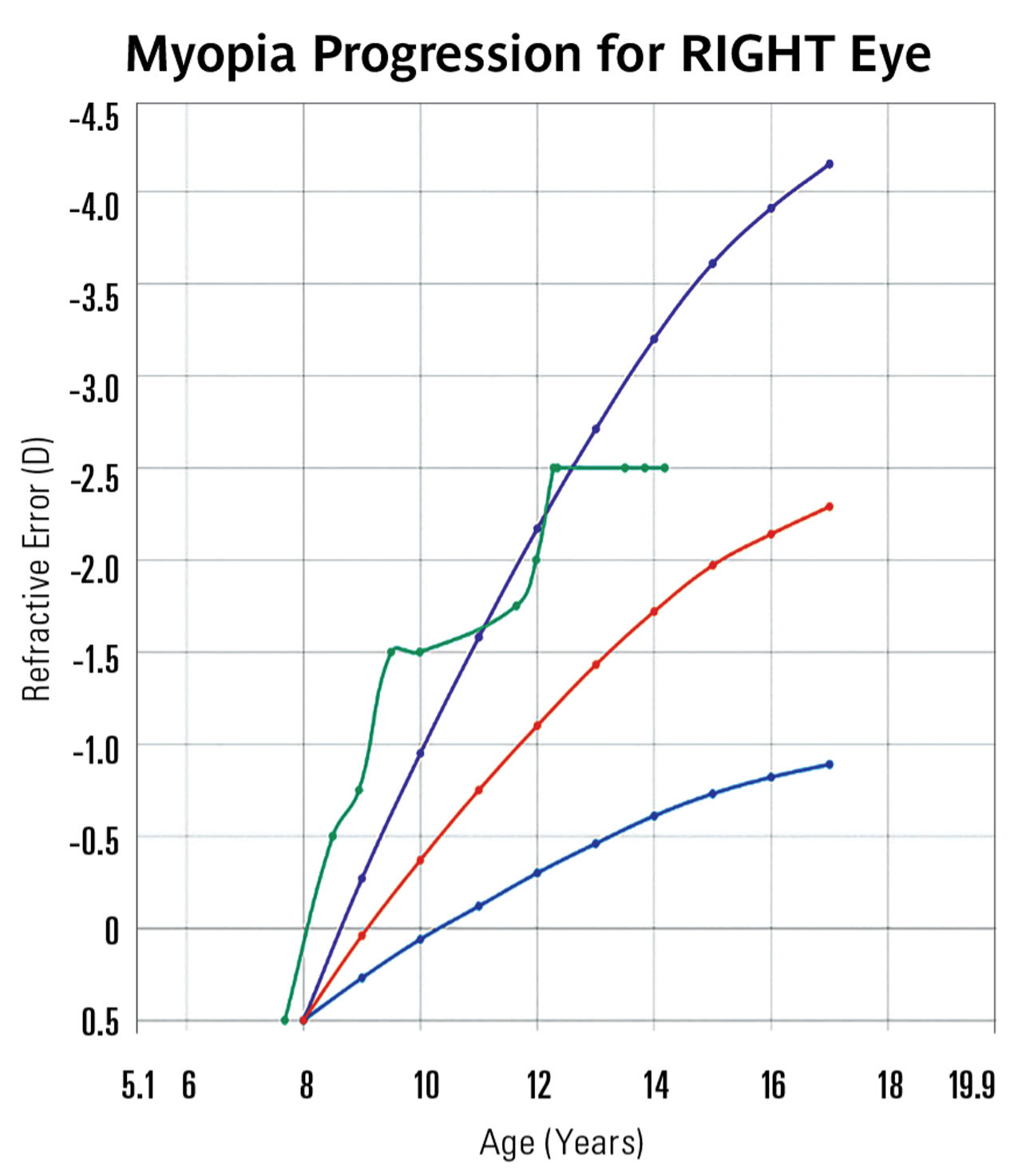
Current research has found variations in the correlation between dioptric change and axial length in millimeters. While 1 study9 found that 1 mm of increase in axial length corresponded with 1.44 D to 1.63 D of change in refraction, another study found 2.50 D to 3.00 D of change with each 1 mm of change in axial length.10
Because axial length is more closely related to potential ocular pathology and dioptric changes have shown variation in their relationship to axial length changes, it is often more effective to explain the changes in axial length when communicating with parents about the rate of myopia progression. However, for practices that do not yet offer axial length measurement, graphing changes in refractive data can still be a powerful tool.
Software, whether embedded in axial length instruments or third-party, can be a handy tool for demonstrating change. For a clinician, it can be easier to see whether a patient’s progression is speeding up or slowing down when looking at these types of tools compared to raw data numbers. When parents can see those changes, it makes them more confident to follow the clinician’s recommendations (Figure 2).

Using the data points from patient exams, AI can be designed (without patient identifiers) to track the percentage of slowing for a given patient—whether the treatment is aligning with “normal” progression or additional myopic dosage should be initiated (Figure 3). Displaying data sets for patients in a visual format makes it easier to explain the condition to parents. When interoperability of medical records and instrumentation becomes more common in the future, these AI programs can add the data directly to the models (Figure 4).
Profilometry for Big Views
While corneal topography continues to serve as a contact lens fitter’s primary device for disease detection and management, the recent introduction of profilometry devices has innovated the corneal and scleral lens world. Most ECPs have seen that over the last 20 years there has been a shift away from spherical scleral lenses to lenses with more advanced peripheral curves. Although spherical scleral lenses have had a dynamic impact on patients who could not otherwise wear lenses, research using profilometry has found that only around 6% of patients have spherical sclerae.11
As scleral lens fitting has grown, manufacturing laboratories have innovated with toric periphery scleral lenses designs. Interestingly, research has shown that toric sclerals only account for around 28% of our patients.12 The remainder of patients (67%) have asymmetric scleral shapes.12 While many patients do well with scleral lenses that have toric peripheries, lenses that can be customized with quadrant-specific shapes—or even customized shapes—are demonstrating great success.
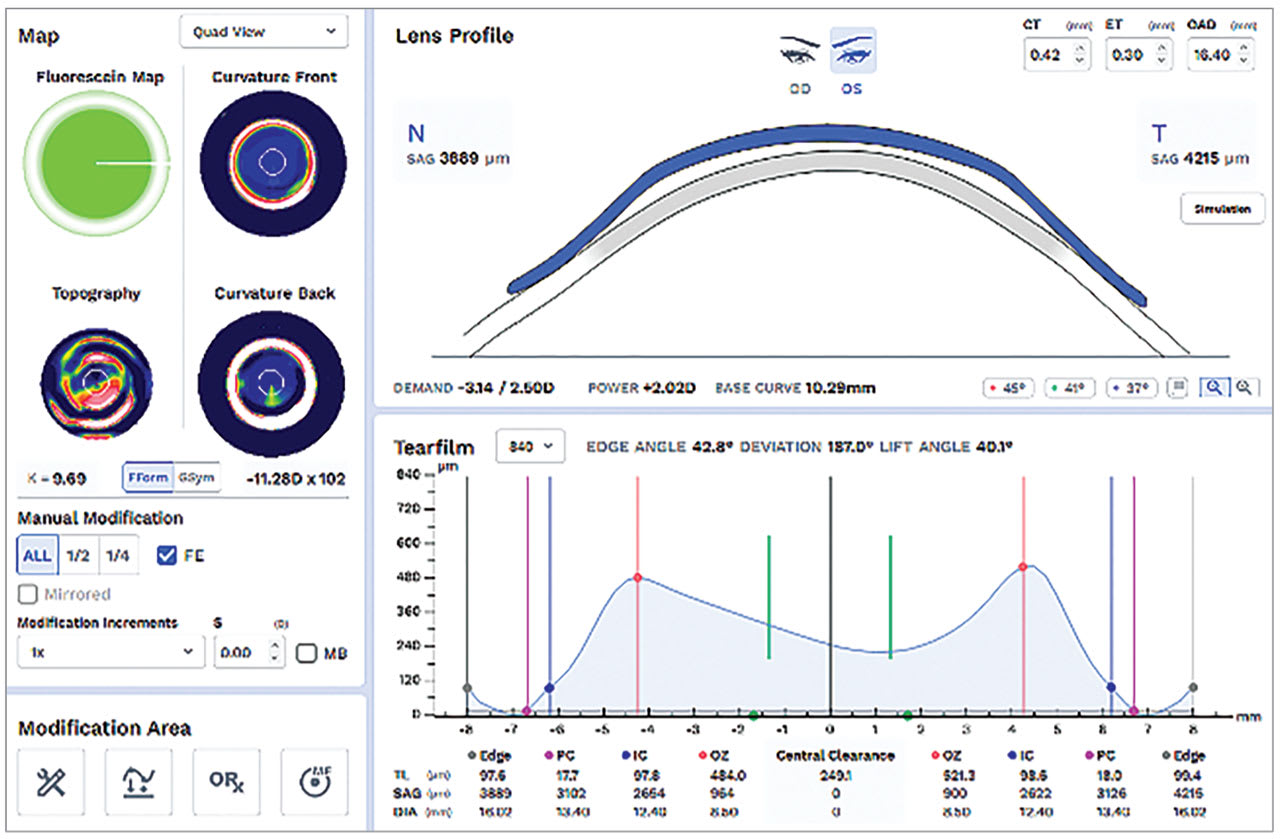
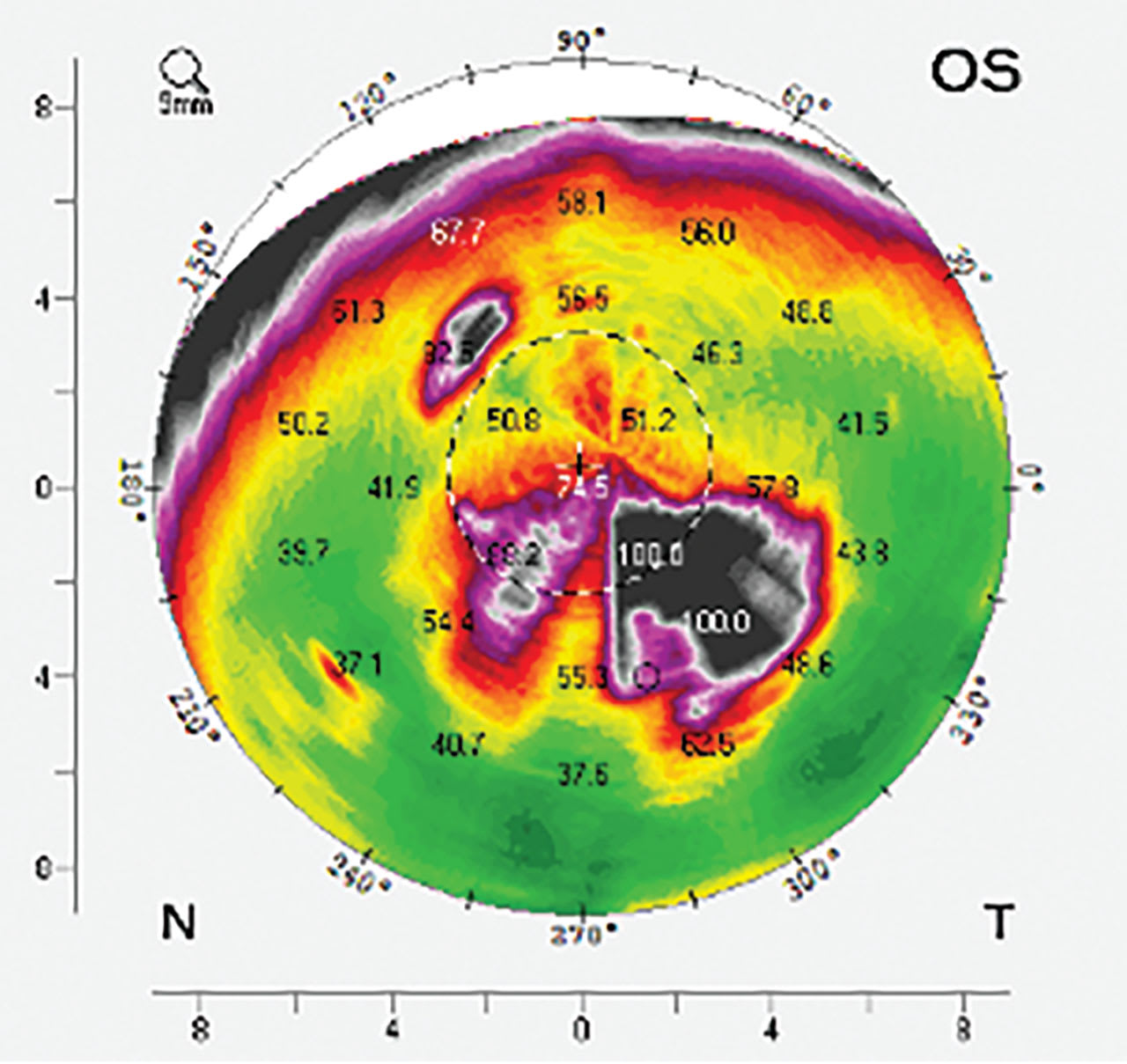
Tomography and topography instruments are available to image scleral contours. Tomography is a Scheimpflug-based system that utilizes cross-sectional measurements taken while rotating around a central point along a given chord of the eye. This data is used to create a data map of the cornea or the entire ocular surface for which the chord is set (Figure 5).

Scleral topography uses reflection-based assessment of the ocular surface.13 Unlike the cornea, the sclera/conjunctiva is not very reflective, so fluorescein is needed. If using this method, make sure to use an ample amount of fluorescein to capture as much of the ocular surface as possible. The system uses a cobalt blue grid to image the eye and then process the shape data.14
With either system, it is essential to capture a very high-quality image; this specific image will be used to design a custom contact lens for the patient. With both systems, manually holding the upper lid out of the way is crucial to obtain information about the superior ocular surface. Whether the image will be used to establish a baseline to monitor progressive changes in the ocular shape or to design contact lenses, investing the time to capture high quality and repeatable images will yield the best results.
Patients who have pinguecula or blebs resulting from glaucoma surgery benefit significantly from a profilometry-guided design. Profilometry is not required for scleral lens fitting; practitioners should also be familiar with scleral fitting techniques and the intricacies of lens design and fitting using slit lamp observation and diagnostic lenses. When managing a large number of patients, having more precise fitting capabilities may pay off.
AI-Guided Lens Fitting
Although AI has yet to make its greatest impact in the world of contact lenses, its use is expanding. Some companies are using AI to design lenses. Several available instruments allow users to send their topography or profilometry data directly to a lens manufacturer. By providing additional data determined by the practitioner, such as the over-refraction found with a specific diagnostic lens or the desired fluid reservoir depth, advanced algorithms (ie, AI) may be able to design an initial lens. The ECP would still have the ability to make modifications to the design, diameter, power, etc, if desired.
Variables—such as lid tightness, which could impact ortho-k wear, or individualized conjunctival compression, which could impact the settling of a scleral, can make a lens change necessary, although future understanding of these factors could be added to AI software. The real beauty of AI is that it gets better and more accurate with improved data points (Figure 6). While the innovations are not yet mainstream, slit lamp cameras and ocular coherence tomography being linked with AI software in the future could help with lens modification after an initial lens has been dispensed.
More Than an Aberration
Practitioners are familiar with the struggles of keratoconus patients who experience optical aberrations—even with best-corrected vision. A 2021 study showed that keratoconus patients who have relatively normal visual acuity still demonstrate elevated higher-order aberrations (HOAs) and reduced contrast sensitivity.15
Having an aberrometry instrument in the office benefits ECPs and patients in 2 main ways. First, the device serves as an educational tool. Practitioners can demonstrate lower-order aberrations (LOAs) and reveal that the patient has a visual problem that may not respond to typical vision correction. This helps patients understand why normal refractive corrections will not work for them and also explains why they are not seeing as well as they may desire. Second, the device may be used to show how much improvement a patient may get with custom contact lenses.16
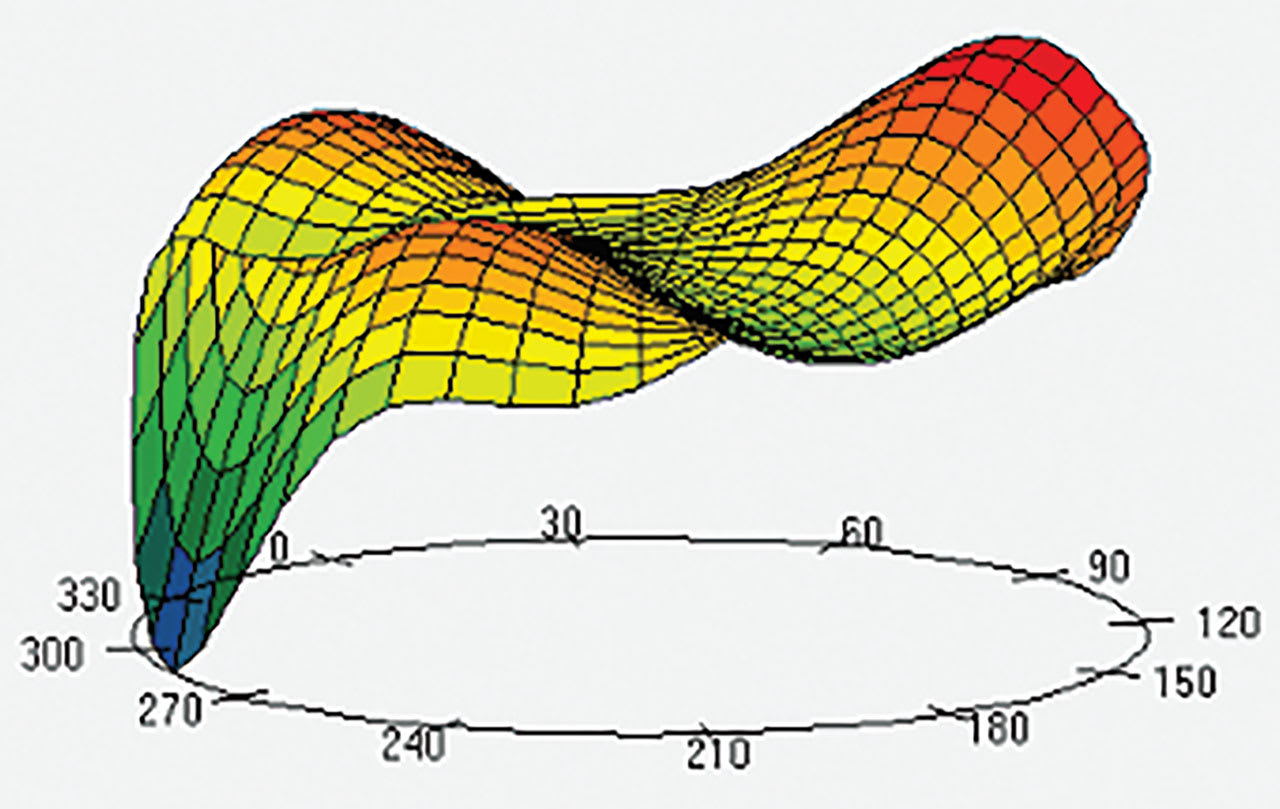
While aberrometry is not yet part of most comprehensive vision correction exams, it is likely that AI will help maximize outcomes by using HOA data in the future (Figure 7). Patients who wear specialty lenses may benefit most from this innovative technological advancement.
Corneal Densitometry When Things Get Cloudy
The slit lamp is the most impactful tool for assessing the ocular surface and contact lenses. Advances in technology can also make it possible to assess the clarity of ocular structures. Corneal densitometry (CD) objectively measures corneal transparency using Scheimpflug imaging. This assessment can be helpful when monitoring various conditions such as corneal edema, inflammation, and scarring (Figure 8). Changes in CD have been associated with changing visual acuity, especially for the central 2 mm to 6 mm of the cornea.17,18
Having an objective measure of the clarity of the cornea can provide a quantifiable measurement to monitor eyes recovering from pathological conditions and may also give patients a better understanding of their condition and their expected outcome. Adding CD to your assessments of HOAs and LOAs can be useful when caring for patients who aren’t satisfied with their best-corrected acuity.
Explosion in Tech Awaits Us
There has been tremendous technological innovation recently. However, it is anticipated that the progress seen in the past 20 years will pale in comparison to what will be accomplished in the next 10 years. For now, using objective and technology-integrative innovations will create a foundation for this explosive breakthrough. Using AI in clinical practice is a great way to maximize your outcomes, efficiency, and patient satisfaction.
References
1. Efron N, Morgan PB, Nichols JJ, et al. All soft contact lenses are not created equal. Cont Lens Anterior Eye. 2022 Apr;45(2):101515. doi: 10.1016/j.clae.2021.101515
2. Barr JT. “Generic” soft contact lenses: scientific, clinical, and regulatory matters. Contact Lens Spectrum. 2021;36(3):37-40. clspectrum.com/issues/2021/march/generic-soft-contact-lenses-scientific-clinical-and-regulatory-matters
3. van der Worp E, Mertz C. Sagittal height differences of frequent replacement silicone hydrogel contact lenses. Cont Lens Anterior Eye. 2015;38(3):157-162. doi: 10.1016/j.clae.2015.01.004
4. van der Worp E, Lampa M, Kinoshita B, Fujimoto MJ, Coldrick BJ, Caroline P. Variation in sag values in daily disposable, reusable and toric soft contact lenses. CLAE. 2021;44(6):101386. commons.pacificu.edu/work/ns/380b99ad-5f0b-4e77-a286-a8d1f74bdfe9
5. Sorbara L, Maram J, Fonn D, Woods C, Simpson T. Metrics of the normal cornea: anterior segment imaging with the Visante OCT. Clin Exp Optom. 2010;93(3):150-1156. doi: 10.1111/j.1444-0938.2010.00472.x
6. Achong-Coan R. How do normal and keratoconic eyes differ in shape? Poster presented at the 2012 Global Specialty Lens Symposium, Las Vegas, Nevada.
7. Tideman JWL, Snabel MCC, Tedja MS, et al. Association of axial length with risk of uncorrectable visual impairment for europeans with myopia. JAMA Ophthalmol. 2016;134(12):1355-1363. doi: 10.1001/jamaophthalmol.2016.4009
8. McCullough S, Adamson G, Breslin KMM, McClelland JF, Doyle L, Saunders KJ. Axial growth and refractive change in white European children and young adults: predictive factors for myopia. Sci Rep. 2020;10(1):15189. doi: 10.1038/s41598-020-72240-y
9. Walline JJ, Lindsley K, Vedula SS, et al; BLINK Study Group. Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: The BLINK randomized clinical trial. JAMA. 2020;324(6):571-580. doi: 10.1001/jama.2020.10834
10. Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G. A 3-Year Randomized Clinical Trial of MiSight Lenses for Myopia Control. Optom Vis Sci. 2019;96(8):556-567. doi: 10.1097/OPX.0000000000001410
11. DeNaeyer G. The shapeshifters. Contact Lens Spectrum. 2023;38(8):20-22,24-26. clspectrum.com/issues/2023/august/the-shape-shifters
12. DeNaeyer G, Sanders D, van der Worp E, Jedlicka J, Michaud L, Morrison S. Qualitative assessment of scleral shape patterns using a new wide field ocular surface elevation topographer: The SSSG study. JCLRS. 2017;1(1):12-22. doi:10.22374/jclrs.v1i1.11
13. DeNaeyer G, Sanders DR, Farajian TS. Surface coverage with single vs. multiple gaze surface topography to fit scleral lenses. Cont Lens Anterior Eye. 2017 Jun;40(3):162-169. doi: 10.1016/j.clae.2017.03.009
14. Jedlicka J. Ocular surface profiling for specialty lens fitting. Contact Lens Spectrum. 2022;37(9): 22-24,26-28,30,31. clspectrum.com/issues/2022/september/ocular-surface-profiling-for-specialty-lens-fitting
15. Shneor E, Piñero DP, Doron R. Contrast sensitivity and higher-order aberrations in keratoconus subjects. Sci Rep. 2021;11(1):12971. doi:10.1038/s41598-021-92396-5
16. Marsack JD. Ravikumar A, Nguyen C, et al. Wavefront-guided scleral lens correction in keratoconus. 2014;91(10):1221-1230. doi: 10.1097/OPX.0000000000000275
17. Lazaridis A, Droutsas K, Sekundo W, Peterak M, Schulze S. Corneal clarity and visual outcomes after small-incision lenticule extraction and comparison to femtosecond laser-assisted in situ keratomileusis. J Ophthalmol. 2017:2017:5646390. doi: 10.1155/2017/5646390
18. Schaub F, Gerber F, Adler W, et al. Corneal densitometry as a predictive diagnostic tool for visual acuity results after Descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2019;198:124-129. doi: 10.1016/j.ajo.2018.10.002