LEARNING METHOD AND MEDIUM
This educational activity consists of a written article and 20 study questions. The participant should, in order, read the Activity Description listed at the beginning of this activity, read the material, answer all questions in the posttest, and then complete the Activity Evaluation/Credit Request form. To receive credit for this activity, please follow the instructions provided below in the section titled To Obtain CE Credit. This educational activity should take a maximum of 2 hours to complete.
CONTENT SOURCE
This continuing education (CE) activity captures key statistics and insights from contributing faculty.
ACTIVITY DESCRIPTION
This article discusses the diverse applications of scleral lenses beyond vision correction.
TARGET AUDIENCE
This educational activity is intended for optometrists, contact lens specialists, and other eyecare professionals.
ACCREDITATION DESIGNATION STATEMENT
This course is COPE accredited for 2 hours of CE credit. COPE Course ID: 98464-CL
DISCLOSURES
Melissa Barnett, OD, has received remuneration from ABB, Abbvie, Alcon, Azura, Bausch + Lomb, BCLA, Bruder, Cloudbreak Pharma, CooperVision, Dompé, Epion, JJVC Vistakon, Lentechs, Ocusoft, Orasis, ScienceBased Health, STAPLE Program, Tarsus, and Théa Pharma. Daddi Fadel, DOptom, has received remuneration from Bausch & Lomb, Boston Materials, Contamac, Eaglet Eye, EasyLac, Mediwork, Medlac, Multilens, Oculus, Topcon Healthcare, and Wave Eyecare.
DISCLOSURE ATTESTATION
The contributing faculty members have attested to the following:
1. That the relationships/affiliations noted will not bias or otherwise influence their involvement in this activity;
2. That practice recommendations given relevant to the companies with whom they have relationships/affiliations will be supported by the best available evidence or, absent evidence, will be consistent with generally accepted medical practice;
3. That all reasonable clinical alternatives will be discussed when making practice recommendations.
TO OBTAIN CE CREDIT
To obtain COPE CE credit for this activity, read the material in its entirety and consult referenced sources as necessary. We offer instant certificate processing for COPE credit. Please take the posttest and evaluation online by using your OE tracker number and logging in to vccecredit.com.
Upon passing the test, you will immediately receive a printable PDF version of your course certificate for COPE credit. On the last day of the month, your course results will be forwarded to ARBO with your OE tracker number, and your records will be updated. You must score 70% or higher to receive credit for this activity. Please make sure that you take the online posttest and evaluation on a device that has printing capabilities.
NO-FEE CONTINUING EDUCATION
There are no fees for participating in and receiving credit for this CE activity.
Disclaimer
The views and opinions expressed in this educational activity are those of the faculty and do not necessarily represent the views of Contact Lens Spectrum. This activity is copyrighted to Conexiant ©2025. All rights reserved.
CE Questions? Contact accred@conexianteducation.com for help.
Release date:JULY 1, 2025
Expiration Date: June 18, 2028
SCLERAL LENS (SL) APPLICATIONS extend beyond conventional fitting or vision correction. SLs offer innovative solutions for a wide range of ocular challenges, including oblate lens designs for keratoconus, and high myopia, optic zone decentration, prism correction, piggyback SLs, drug delivery, low-vision rehabilitation, and safety protection. Expanding expertise in SLs will allow practitioners to utilize them to their full potential, maximize patient outcomes, and consider alternative solutions when conventional management strategies prove insufficient.
1. Oblate Design for Prolate Corneas to Reduce High-Minus Power
Oblate designs, also known as reverse geometry, feature a back optic zone radius (BOZR) that is flatter than the adjacent peripheral curve. Oblate designs may be used for prolate corneas to reduce high-minus power. The BOZR determines the lens power, and flattening the BOZR results in a lens with less minus (or more plus) power.
High-powered SLs tend to be thicker and heavier, making them more likely to decenter. This decentration can negatively affect fit, comfort, and quality of vision. Flattening the BOZR to reduce the minus power reduces lens thickness and mass, allowing for improved centration and vision.
While lens power alone does not directly induce coma, their increased weight makes them more likely to decenter, which in turn amplifies the impact of decentration on coma. In contrast, plano or low-powered lenses are thinner and lighter, making them less prone to decentration and, therefore, less likely to induce coma.
While coma is mainly affected by lens decentration, spherical aberration is more influenced by lens power. High-minus lenses create a greater negative spherical aberration.1 In an oblate design, the central light rays focus behind the peripheral rays, resulting in a positive shift in spherical aberration.2In high myopia and advanced corneal ectasia, using a reverse-geometry design with a flatter BOZR will lessen the positive shape of the tear lens. This results in an SL that requires less minus power, which affects image magnification and improves oxygen transmission in myopic corrections.2
Lenses that have a high negative power generally require a smaller back optic zone diameter (BOZD). In cases in which the lens decenters, the junction between the optic zone and the adjacent peripheral curve may fall within the pupil area. This misalignment can degrade visual quality by causing glare, halos, and reduced contrast sensitivity. This visual disturbance is particularly noticeable in individuals who have large pupil diameters or in low-light conditions, when the pupil dilates. Proper lens design, centration assessment, and pupil size consideration are essential to minimize these unwanted optical effects and optimize visual performance. Reducing lens negative power with an oblate design will allow for a larger BOZD, minimizing the effects of decentration and the optic zone junction falling within the pupil.
Using an oblate lens design is especially useful in cases of keratoconus when the cone is not in the center of the cornea. When fitting a lens for keratoconus, it is essential to ensure sufficient clearance over the cone apex to avoid mechanical stress on the cornea, potential scarring, and discomfort. An oblate design will allow vaulting of the midperipheral cornea without creating excessive central fluid reservoir depth.
2. Back Optic Decentration for Fitting Purposes
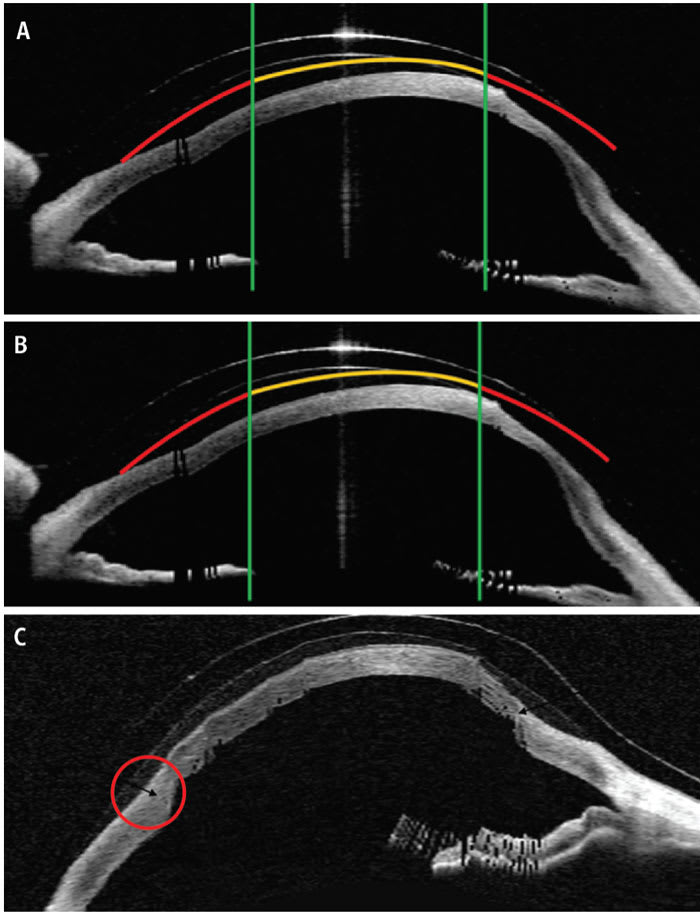
Although an oblate design can provide increased clearance over a non-central cone and optimal clearance over the central cornea, it may lead to excessive superior clearance that can compromise both the lens fit and corneal metabolism. Decentering the optic zone helps achieve better balance by optimizing clearance over the inferior cone while maintaining appropriate central clearance and reducing excessive superior clearance (Figure 1).
3. Front-Surface Optic Modifications
When fitting scleral lenses, customizing the front optic zone can enhance visual performance, especially in patients who have irregular corneas and higher-order aberrations (HOAs). Adding toricity to correct residual astigmatism is a common front-surface modification when assessing a rotationally stable lens. Other front-surface customization options include adding wavefront-guided optics, front-surface eccentricity, and multifocal optics.
Wavefront-guided optics improve optical performance beyond the usual spherical and toric corrections. Irregular corneas often cause significant coma, trefoil, and spherical aberrations. Wavefront-guided optics compensate for these HOAs and improve contrast sensitivity, night vision, and overall vision quality and decrease glare and halos.3Decentering wavefront-guided corrections on the front surface of a scleral lens to align with the HOAs can provide improved acuity.4
High-refractive errors and irregular corneas typically exhibit spherical aberrations, which may be addressed by adding front-surface eccentricity. This improves visual fatigue and depth of field and enhances vision clarity in different light conditions. The front surface can also be modified to include multifocal optics for presbyopic patients to enable simultaneous vision correction at several distances.
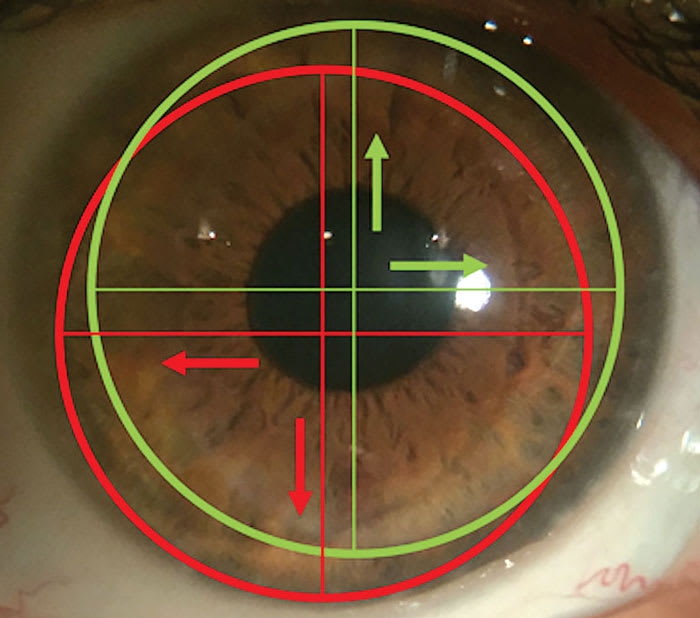
The optical performance of SLs must take into consideration pupil size and location, lens decentration, and corneal abnormalities. Therefore, front optic zone customizations should be aligned with the patient’s visual axis rather than the geometric center of the SL (Figure 2). Misalignment can cause residual aberrations, fluctuating vision, decreased contrast sensitivity, and non-optimal vision quality.
4. Prism to Correct Tropias
Scleral lenses have been shown to correct diplopia due to strabismus by incorporating a prism into the optic zone.5,6,7In a collaborative case, a professional soccer player with 10 ∆ intermittent exotropia was successfully fitted with 5 ∆ prism diopter scleral lenses in each eye, providing stability and excellent comfort during sports activities.8 Correcting strabismus using prism can be challenging due to difficulties in maintaining lens rotational stability.
Interestingly, Sherafat and colleagues found that in 20 asymmetric keratoconus patients, SLs alone improved binocularity without prism in most cases.9 These findings suggest assessing binocularity with standard SLs before adding prism correction.9
5. Piggyback Lenses
A piggyback system in which a soft contact lens is placed under an SL may enhance fitting success in patients who have handling difficulties or anatomical obstacles. While corneal edema is a concern with both soft and scleral lenses, a recent study has shown similar levels of central corneal edema in standard SL wear and piggyback systems.10
Fitting an SL over a tinted soft lens was shown to alleviate photophobia in patients who had severe ocular surface disease.11 This option may also be used for aniridia and for cosmetic purposes.
Handling difficulties with SLs can lead to frustration and dropout. A soft lens may be used as a training tool to build confidence before transitioning to an SL, particularly with pediatric patients. In cases where discomfort persists, a piggyback approach can improve tolerance until the SL can be worn independently.12
For patients who have allergies to rigid lens materials, placing a soft lens beneath the SL to minimize direct contact may reduce allergic reactions.13 This can also alleviate discomfort and improve lens stability in patients who have conjunctival irregularities, such as pinguecula, pterygium, or symblepharon. Symblepharon can lift the SL edge and cause decentration, air bubbles, or displacement. A piggyback enhances fit, comfort, and retention, making it a valuable solution for these conditions.14
Piggybacking may also reduce fluid reservoir debris, commonly known as midday fogging by decreasing post-lens fluid volume and acting as a barrier against debris.14,15 Midday fogging has been reported in 26% to 46% of patients and can negatively impact visual clarity.16 While the exact cause and composition of the debris remain uncertain, it has been linked to lipids,17 leukocytes,16 and external tear film contaminants.18 Patients may experience foggy or blurred vision either shortly after lens application or after several hours of lens wear.18
Piggybacking can also help reduce conjunctival prolapse by stabilizing the lens fit and preventing excessive conjunctival bulging into the limbal area.13
A reverse piggyback system has been used for pediatric patients who cannot tolerate patching.19 Their visual development can be supported by placing an occlusive soft lens over an SL, although care must be taken to educate parents and caregivers to reduce infection risks. Reverse piggybacking also improves lens wettability, especially when lenses are stored dry, by enhancing tear film stability and reducing poor wetting areas that affect vision.13
6. Ptosis
Ptosis is frequently treated by surgical correction. New treatment options include oxymetazoline hydrochloride pharmaceutical drops.20 It has been suggested that SLs can also be a noninvasive, reversible, and conservative management approach to treating ptosis without the risks associated with anesthesia, incisions, or postoperative recovery.21
A large-diameter SL covers a wide surface area with an elevated sagittal height, leading to an eyelid lift and improving eyelid cosmesis.21 Publications on managing ptosis with SLs are limited. Katsoulos and colleagues reported that fitting an SL that had an elevated central clearance in a pediatric patient led to higher upper eyelid support and a slightly more open palpebral aperture.22 Cherny and coworkers reported the case of a 28-year-old patient who had chronic progressive external ophthalmoplegia who gained more than 3.0 mm of improvement with SLs.23 SLs for ptosis management have been reported to improve cosmesis, visual acuity, symptoms of dry eye, and patient-reported self-esteem.21
When prescribing SLs for ptosis, patient selection, eyelid anatomy, and lens design must be considered. Collaborative care between cornea and contact lens specialists and oculoplastic surgeons may yield the best outcomes, particularly in complex cases or when surgery is not indicated.
7. Drug Delivery
Traditional topical therapies for dry eye, such as eye drops, are limited by low bioavailability, rapid tear turnover, and poor patient adherence.24 As interest in innovative ocular drug delivery systems grows, SLs are becoming a promising platform for sustained and localized delivery of therapeutics to both the anterior and posterior segments of the eye.
Although SLs are off label for this use, researchers have found that they offer multiple advantages as a drug-delivery platform because they enhance retention time.24 Unlike conventional drops, which are rapidly cleared from the ocular surface, drugs delivered via SLs remain in contact with the eye for extended periods.24 The fluid reservoir between the lens and the cornea enables prolonged drug exposure and absorption and improved bioavailability. Continuous release into the lens reservoir offers a more stable pharmacokinetic profile than intermittent drops provide.24
SLs are also being explored as therapeutic devices for advanced ocular surface disease management. Using SLs to deliver cyclosporine 0.05% was well tolerated, as evidenced by improvements in ocular surface signs and symptoms.25
SLs have been reported to support the healing of persistent epithelial defects by acting as a therapeutic platform for the delivery of nonpreserved or fortified antibiotics via the scleral reservoir.26Studies have shown that they improve corneal neovascularization when anti-VEGF agents are used within the fluid reservoir.27
Stem cell-laden SL carriers provide a novel regenerative approach to treating chemical burns. SLs coated with limbal stem cells have been shown to support epithelial repair and regeneration in animal models.28 As technology advances, SLs present a promising option for managing chronic and complex anterior-segment diseases.
8. Low Vision
Low-vision SLs are emerging as a valuable and aesthetically attractive tool in low-vision management due to advanced design integration.2 Innovations such as telescopic optics within the lens and computer-assisted customization are expanding opportunities for individuals who have severe vision loss. When fitting these lenses, careful consideration of oxygen transmissibility remains essential to ensure long-term corneal health.
9. Protection
Beyond vision correction, literature supports the use of SLs to shield the ocular surface from physical impact and particulate matter.29,30 This makes them particularly valuable for individuals who have fragile or thin corneas, as seen in advanced keratoconus, where the cornea is highly compromised and at risk of mechanical stress and further deterioration. Literature supports the use of SLs in shielding the ocular surface from physical impact and particulate matter.29,30 They are also beneficial in settings that involve increased risk, including sports, dusty work environments, and military training and deployments.
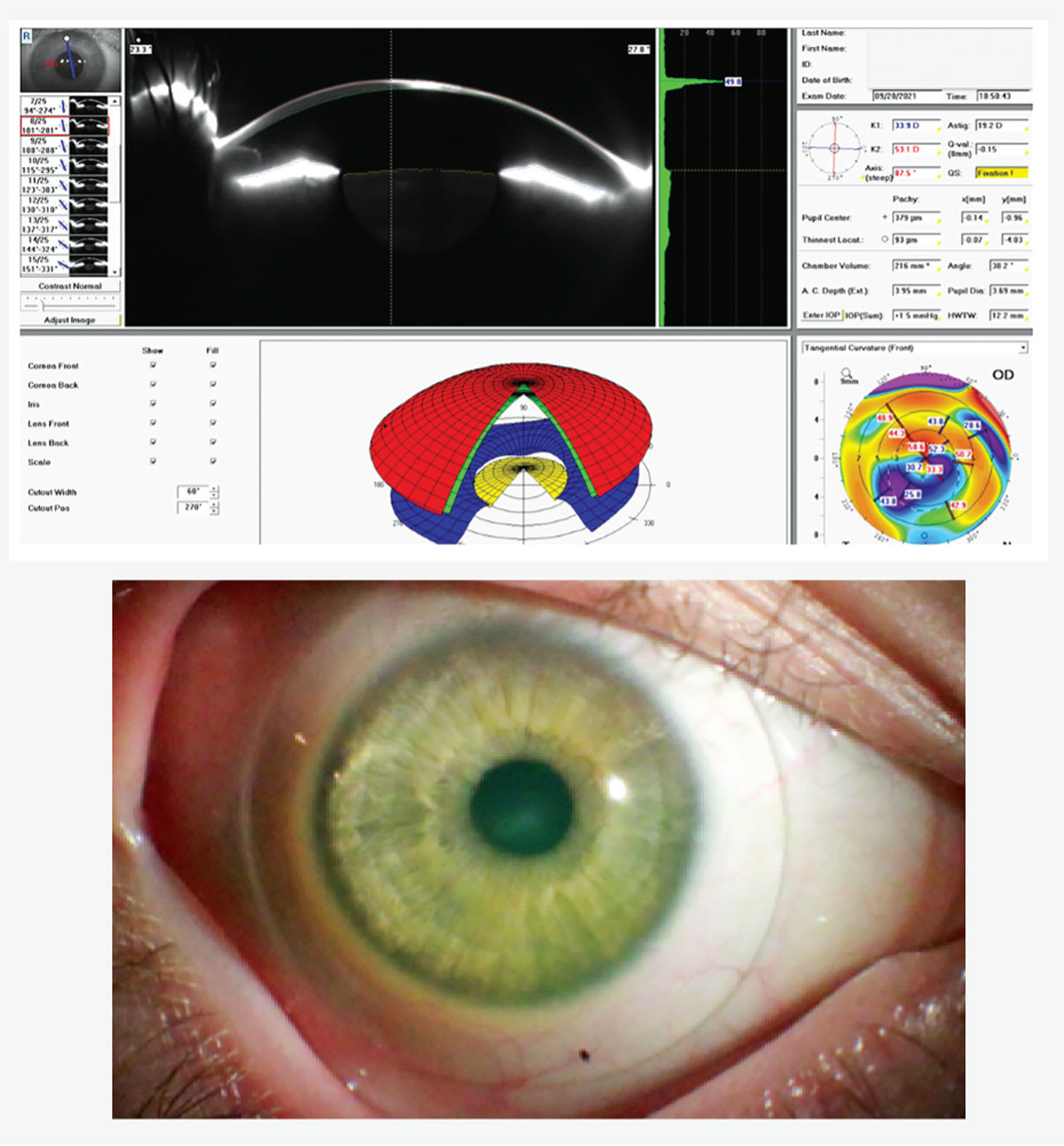
SLs protect fragile corneas, especially in cases of extreme thinning.13 A patient who has a corneal thickness of 93 µm and significant scleral asymmetry benefitted from custom SLs, which acted as a shield protecting the thin and fragile cornea (Figure 3).
SLs have been shown to protect the eye from trauma by absorbing and distributing impact energy, reducing the risk of severe corneal damage.29,30 Walker and colleagues reported a case in which a staple from an electric stapler struck a patient’s eye, creating a hole in the center of the SL.29 The patient experienced moderate pain and conjunctival redness, but the cornea remained unharmed due to the rigidity of the lens and the cushioning effect of the post-lens fluid reservoir.
Similarly, Macedo and colleagues documented a case in which a high-speed object impacted an eye wearing an SL, causing the lens to break without leading to corneal injury.30 Both case studies concluded that the combination of a rigid lens structure and a deep tear reservoir protects the cornea and anterior segment from significant trauma.
Unlike smaller lenses, SLs extend beyond the limbus and rest on the sclera, offering full coverage of the cornea and partial coverage of the conjunctiva. Their large diameter and vaulting design create a sealed reservoir of fluid between the lens and the eye, helping to prevent irritation and dryness from dust, debris, allergens, and wind. This makes them particularly beneficial for individuals working or spending time in construction zones, agricultural fields, military settings, or arid climates, where airborne particles are common.
With the rapid evolution of SL materials and designs, staying up to date on these advancements is essential for optimizing outcomes and ensuring the best possible care for patients requiring specialized contact lens solutions.
References
1. Charman W. Optical aberrations and the eye. Optician. 2003;5898(25):18-23.
2. Vincent SJ, Fadel D. Optical considerations for scleral contact lenses: A review. Cont Lens Anterior Eye. 2019;42(6):598-613. doi:10.1016/j.clae.2019.04.012
3. Marsack JD, Ravikumar A, Nguyen C, et al. Wavefront-guided scleral lens correction in keratoconus. Optom Vis Sci. 2014;91(10):1221-30. doi:10.1097/OPX.0000000000000275
4. Rijal S, Hastings GD, Nguyen LC, Kauffman MJ, Applegate RA, Marsack JD. The impact of misaligned wavefront-guided correction in a scleral lens for the highly aberrated eye. Optom Vis Sci. 2020;97(9):732-740. doi: 10.1097/OPX.0000000000001577
5. Bragg TL, Sindt CW. Correction of binocular diplopia with novel contact lens technology. J AAPOS. 2015;19(4):e38. doi.org/10.1016/j.jaapos.2015.07.113
6. Frogozo M. Treatment of horizontal diplopia with prism correction in scleral gas permeable prosthetic device. 2016. sclerallens.com/PDFs/Frogozo.pdf
7. Lee SK, Zabrowski C, McClelland CM, Lee MS. Treatment of horizontal binocular diplopia with prismatic contact lenses. J Neuroophthalmol. 2021 Mar 1;41(1):e81-e82. doi: 10.1097/WNO.0000000000000936
8. Fadel D. Scleral Lenses Issues and Complications: Etiology, Recognition and Management. Dougmar Publishing Group Inc. Ontario, Canada; 2020.
9. Sherafat H, White JE, Pullum KW, Adams GG, Sloper JJ. Anomalies of binocular function in patients with longstanding asymmetric keratoconus. Br J Ophthalmol. 2001;85(9):1057-60. doi:10.1136/bjo.85.9.1057
10. Bliss VH, Branjerdporn N, Ooi PJ, et al. Corneal oedema during reverse piggyback scleral lens wear. Ophthalmic Physiol Opt. 2023;43(5):1065-1069. doi:10.1111/opo.13161
11. Subramanian M, Balaji J. Piggyback scleral contact lens to enhance cosmesis and comfort in uniocular stevens-johnson syndrome. Eye Contact Lens. 2025;51(3):e157-e159. doi:10.1097/ICL.0000000000001143
12. Murphy DA, Samples JS, Zepeda EM, Riaz KM. Progression from soft lens to piggyback soft-scleral contact lens system to facilitate scleral lens use in a pediatric patient. Eye Contact Lens. 2021;47(7):426-428. doi:10.1097/ICL.0000000000000776
13. Fadel D. Three of a kind. Contact Lens Spectrum. 2023:38(7):32-36,38-40, 51. Accessed June 6, 2025. clspectrum.com/issues/2023/july/three-of-a-kind
14. Qiu S, Fadel D, Bray C. Thinking outside of the box: piggybacking scleral lenses to combat midday fogging. Poster presented at the Global Specialty Lens Symposium; 2025; Las Vegas. Accessed June 6, 2025. gslsymposium.com/live/2/page/59?poster_id=235
15. Barnett M, Courey C, Fadel D, et al. CLEAR - scleral lenses. Cont Lens Anterior Eye. 2021;44(2):270-288. doi:10.1016/j.clae.2021.02.001
16. Postnikoff CK, Pucker AD, Laurent J, Huisingh C, McGwin G, Nichols JJ. Identification of leukocytes associated with midday fogging in the post-lens tear film of scleral contact lens wearers. Invest Ophthalmol Vis Sci. 2019;60(1):226-233. doi:10.1167/iovs.18-24664
17. Walker M, Morrison S, Caroline P, et al. Laboratory analysis of scleral lens tear reservoir clouding. Poster presented at the 2014 Global Specialty Lens Symposium; 2014; Las Vegas.
18. Walker MK, Bergmanson JP, Miller WL, Marsack JD, Johnson LA. Complications and fitting challenges associated with scleral contact lenses: A review. Cont Lens Anterior Eye. 2016;39(2):88-96. doi:10.1016/j.clae.2015.08.003
19. Michaud L, Carrasquillo K. Piggyback cosmetic contact lens as an occlusion therapy in a patient with familial dysautonomia. Eye Contact Lens. 2010;36(6):367-70. doi:10.1097/ICL.0b013e3181f57aed
20. Slonim CB, Foster S, Jaros M, et al. Association of oxymetazoline hydrochloride, 0.1%, solution administration with visual field in acquired ptosis: a pooled analysis of 2 randomized clinical trials. JAMA Ophthalmol. 2020;138(11):1168-1175. doi:10.1001/jamaophthalmol.2020.3812
21. Walter Sherman S. Ptosis Focus. Contact Lens Spectrum. 2023;38(3):46. Accessed June 6, 2025. clspectrum.com/issues/2023/march/the-scleral-lens-vault
22. Katsoulos K, Rallatos GL, Mavrikakis I. Scleral contact lenses for the management of complicated ptosis. Orbit. Jun 2018;37(3):201-207. doi:10.1080/01676830.2017.1383475
23. Cherny C, Sherman SW, Dagi Glass LR. Severe chronic progressive external ophthalmoplegia-associated ptosis successfully treated with scleral lenses: response. J Neuroophthalmol. 2021;41(1):137-138. doi:10.1097/WNO.0000000000001066
24. Novack GD, Barnett M. Ocular drug delivery systems using contact lenses. J Ocul Pharmacol Ther. 2020;36(8):595-601. doi:10.1089/jop.2020.0024
25. Nakhla MN, Patel R, Crowley E, Li Y, Peiris TB, Brocks D. Utilizing PROSE as a drug delivery device for preservative-free cyclosporine 0.05% for the treatment of dry eye disease: a pilot study. Clin Ophthalmol. 2024;18:3203-3213. doi:10.2147/OPTH.S487369
26. Jacobs DS. Update on scleral lenses. Curr Opin Ophthalmol. 2008;19(4):
298-301. doi: 10.1097/ICU.0b013e328302cc4f
27. Bian Y, Jacobs DS. Drug delivery in PROSE device as alternative to frequent drop administration in severe ocular surface disease. Eye Contact Lens. 2025;51(4):206-208. doi: 10.1097/ICL.0000000000001163
28. Espandar L, Caldwell D, Watson R, Blanco-Mezquita T, Zhang S, Bunnell B. Application of adipose-derived stem cells on scleral contact lens carrier in an animal model of severe acute alkaline burn. Eye Contact Lens. 2014;40(4):243-247. doi:10.1097/ICL.0000000000000045
29. Walker M, Caroline P, Kinoshita B, et al. The Protective Advantage of Scleral Contact Lenses. Poster presented at the Global Specialty Lens Symposium; 2015; Las Vegas.
30. Macedo-de-Araujo RJ, van der Worp E, Gonzalez-Meijome JM. On-eye breakage and recovery of mini-scleral contact lens without compromise for the ocular surface. Cont Lens Anterior Eye. 2018;41(3):311-314. doi:10.1016/j.clae.2017.12.009




