AT THIS PAST January’s International Forum for Scleral Lens Research, preliminary data on anterior chamber (AC) changes during scleral lens (SL) wear were presented. Shortly afterward, a study was published that provided an in-depth analysis of this topic (Jiang et al, 2025). This study shows that SL-induced compression affects not only the conjunctiva, as previously thought, but also the entire AC structures, where we least expected it—fascinating.
The authors of this study assessed the impact of SLs in the short term (0 to 4 hours) on a population of Asian wearers (18 to 35 years). They analyzed the following parameters via anterior segment optical coherence tomography (AS-OCT) images: anterior chamber depth (AD), angle opening distance at 500 µm (AOD500), and trabecular-iris space area at 500 µm (TISA500) as well as central corneal thickness (CCT).
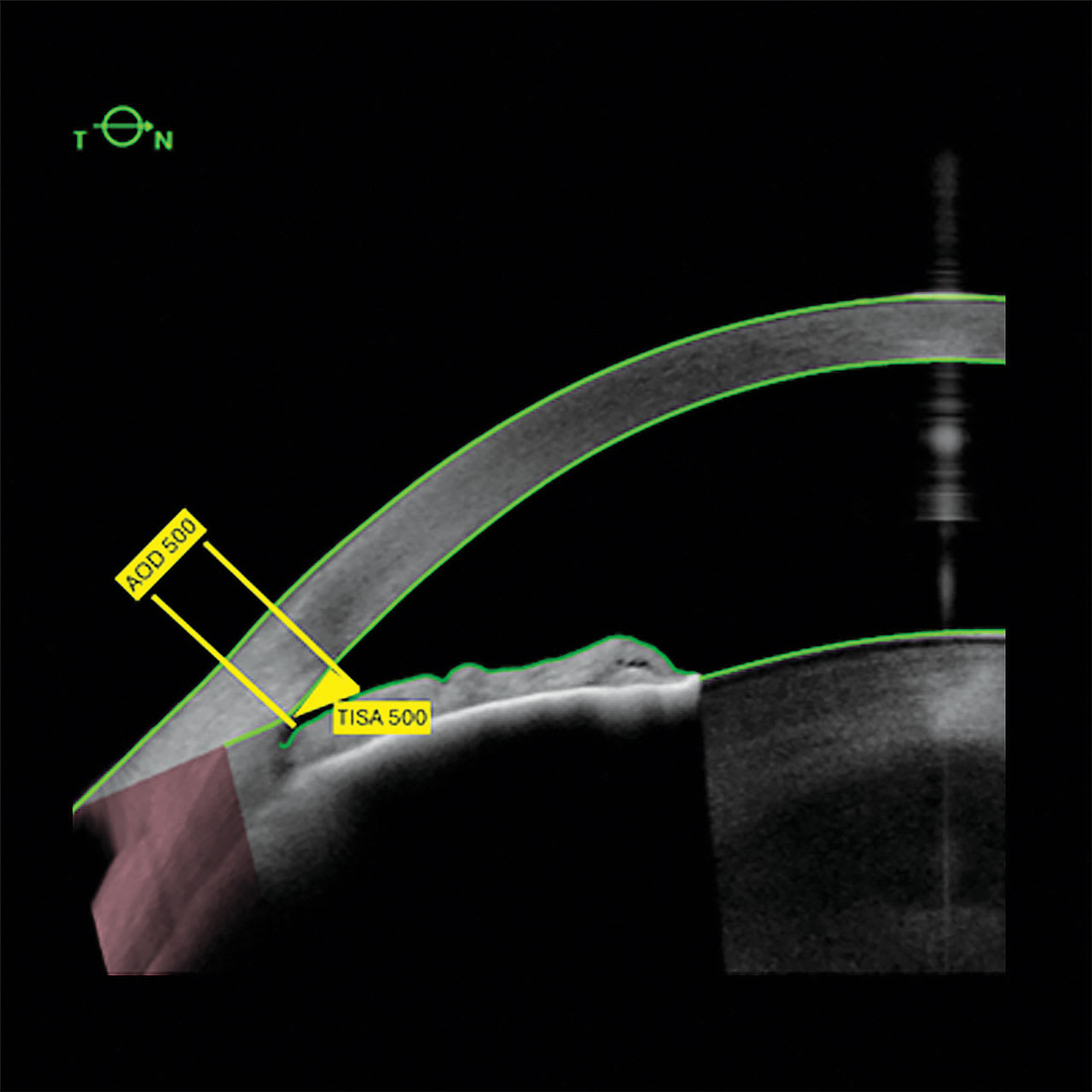
AOD500 is defined as the perpendicular distance established between the iris surface and the trabeculum, at a point 500 µm anterior to the scleral spur (SS). TISA500 consists of a trapezoidal zone between AOD500 and a perpendicular established between the inner sclera and the opposite iris (Figure 1). These parameters are linked and characterize the opening of the iridocorneal angle.
The SLs used were individually adapted. They had a large diameter (horizontal visible iris diameter [HVID] +3.5 mm) and toric peripheries. The vault over the limbus avoided touching. The lenses were made with high-permeability material (hexafocon A, 100 Fatt units). Fluid reservoir thickness varied optimally (200 µm to 300 µm initially).
Study results showed that AD, AOD500, and TISA500 were all compressed during 4-hour lens wear, while CCT increased by 2.29%. All these parameters quickly returned to baseline when SLs were removed.
These data suggest that compression secondary to SL wear also affects all AC structures. Compressing the AC restricts outflow. This is in addition to the already-documented compression of the episcleral veins and Schlemm’s canal (Vincent et al, 2017; Nau, 2016). This leaves the secondary pathways (uveoscleral, trans-scleral, or uveolytic outflow) unable to compensate fully (Costagliola et al, 2020). Research has found that ocular structures quickly reposition themselves when the lenses are removed (Samaha and Michaud, 2021; Michaud et al, 2025; Cheung et al, 2020).
Research, therefore, helps practitioners to understand the dynamics that develop during SL wear, and may prompt us to exercise caution when fitting patients treated for glaucoma or at risk (narrow angles, ocular hypertension, etc). If there are other lens options, they should be seriously considered. Promoting tear exchange under the lens (channels) or limiting the suction effect by adding fenestrations are associated with mixed outcomes and may not offer enough relief (Fisher et al, 2024).
Take home: Practitioners must consider the ocular health of an eye fitted with an SL in its entirety.It is important to bear this in mind when practitioners explore lens options for their patients.
References
1. Jiang Q, Zhang Z, Niu L, et al. Changes in anterior segment after short-term scleral lens wear in healthy Chinese population. Cont Lens Anterior Eye. 2025;48(1):102291. doi: 10.1016/j.clae.2024.102291
2. Vincent SJ, Alonso-Caneiro D, Collins MJ. Evidence on scleral contact lenses and intraocular pressure. Clin Exp Optom. 2017;100(1):87-88. doi: 10.1111/cxo.12448
3. Nau CB, Schornack MM, McLaren JW, Sit AJ. Intraocular pressure after 2 hours of small-diameter scleral lens wear. Eye Contact Lens. 2016;42(6):350-353. doi: 10.1097/ICL.0000000000000214
4. C. Costagliola, R. Dell Omo, L. Agnifili, et al. How many aqueous humor outflow pathways are there? Surv Ophthalmol. 2020;65(2):144-170. doi: 10.1016/j.survophthal.2019.10.002
5. Samaha D, Michaud L. Bruch membrane opening minimum rim width changes during scleral lens wear. Eye Contact Lens. 2021;47(5):295-300. doi: 10.1097/ICL.0000000000000750
6. Michaud L, Balourdet S, Samaha D. Variation of Bruch’s membrane opening in response to intraocular pressure change during scleral lens wear, in a population with keratoconus. Ophthalmic Physiol Opt. 2025;45(2):405-415. doi: 10.1111/opo.13431
7. Cheung SY, Collins MJ, Vincent SJ. The impact of short-term fenestrated scleral lens wear on intraocular pressure. Cont Lens Anterior Eye. 2020;43(6):585-588. doi: 10.1016/j.clae.2020.02.003
8. Fisher D, Collins MJ, Vincent SJ. Contact lens fenestrations and channels in relation to tear exchange and corneal oedema. Clin Exp Optom. 2024 Nov 20:1-14. doi: 10.1080/08164622.2024.2426823
9. Ytteborg J, Dohlman Ch. Corneal edema and intraocular pressure: II. Clinical results. Arch Ophthalmol.1965;74(4):477-484. doi: 10.1001/archopht.1965.00970040479008



