WITH ANY DISEASE, it is critical not just to treat symptoms or clinical findings but also to consider the underlying or “root” etiology. This process can ultimately lead to the most effective treatment. Patients who have symptoms of ocular discomfort are often initially assumed to have dry eye disease. When encountering these patients, it is important to consider all etiologic factors and comorbidities for dry eye disease that are minimally or nonresponsive to early therapies.
Some underlying etiologies that cause ocular surface dryness can be relatively straightforward to determine with careful examination and a thorough history. These include eyelid and conjunctival abnormalities, infections, allergies, and corneal dystrophies. Others, such as environmental irritants, stem cell deficiency, medication toxicity, systemic rheumatological conditions, and neurologic abnormalities, are notably more difficult to elucidate.1
Neural conditions that can present similarly to (and often overlap with) dry eye are some of the most difficult to understand, diagnose, and treat.1 As understanding of the importance of neural dysfunction grows, however,2 the distinct conditions of neurotrophic keratitis and neuropathic corneal pain are becoming better understood.
By Definition
Neurotrophic disease involves a dysfunction of or damage to a neural network that provides “nutritional” innervational support of tissue via neurotrophic factors.2 This results in hypoesthesia, or reduced corneal nerve function, causing tissue damage. The term “neuropathic” refers to dysfunction of or damage to the nerves that causes anomalous pain signaling.3 The resulting anomaly results in hyperesthesia, or increased corneal nerve function, which increases pain perception.
From a clinical perspective, patients who have neurotrophic disease report less discomfort than one would expect, given their clinical appearance (“stain without pain”), and neuropathic patients report pain that is more severe than clinical signs would predict (“pain without stain”).4
Clinical Similarities
While the conditions may seem to be opposites, they do share similarities at an anatomic level. Broadly speaking, they both result from neural insult somewhere along the pathway that eventually affects the nasociliary branch of the ophthalmic division of the trigeminal nerve.5,6 Confocal microscopy has shown neural abnormalities and decreased nerve density in both conditions, although the instrumentation required for this is not readily available in most primary care clinics.5,6 The list of potentially nerve-damaging etiologies is extensive for both, but there are many overlapping possibilities.
Shared ocular etiologies for both neurotrophic keratitis and neuropathic corneal pain include infectious disease (herpetic being very common),7 dry eye disease, chronic topical medication use, topical anesthetic abuse, and contact lens wear. Trauma to the trigeminal nerve or its peripheral extensions via cerebrovascular accidents, malignancy, palsy, surgical procedures (including corneal surgeries, both refractive and therapeutic), or chemotherapy and radiation can lead to neurotrophic keratitis and/or neuropathic pain.
Diabetes is a common systemic etiology of both neuropathic disease and neuropathic pain.5,6 Etiologies unique to neurotrophic disease include corneal dystrophies, vitamin A deficiency, and rare congenital dysautonomia disorders. Conditions more unique to neuropathic pain include small-fiber polyneuropathy, fibromyalgia, trigeminal neuralgia, and autoimmune disease.5,6,8
The symptoms reported by both these types of patients are often similar to each other and to those of the dry eye patient. These patients frequently report a long history of previously attempted but ineffective traditional dry eye therapies. They may report varying degrees of discomfort and pain, dryness, foreign body sensation, burning, blurry vision, and/or photophobia.5,6
It is important to continue to treat any ocular surface disease and dryness to preserve and protect the ocular surface. Furthermore, chronic dry eye has been suggested as a possible cause of neural damage and abnormal corneal sensitivity in both hyper and hypo directions.9 In both cases, however, traditional dry eye therapies are rarely enough to mitigate the neural dysfunction that is driving the patient’s pathology.
Clinical Differences
Despite any similarities in symptoms, anatomic pathway, and inciting etiologies, there can be stark differences in the clinical presentation of these 2 conditions.
Neurotrophic keratopathy: This results from insufficient corneal innervation that impairs lacrimal secretion, epithelial cell metabolism, and release of trophic factors that promote healing. This disrupted corneal homeostasis results in poor healing, corneal epithelial breakdown, reduced tear film, and reduced blink rate.5
In 2023, The Neurotrophic Keratopathy Study Group proposed a new classification system that describes 6 stages based on progressing clinical signs.5 All stages include decreased corneal sensation. Stages 1 and 2 have zero to mild punctate epithelial keratopathy (Figure 1), stage 3 has persistent/recurrent epithelial defects (Figure 2), as does stage 4 but with the addition of stromal haze, and stage 5 adds corneal ulceration that can progress to perforation in stage 6.5 With worsening clinical appearance, patients often report fewer symptoms of pain as their corneal sensation deteriorates, which can reduce compliance with medical treatment and follow-up recommendations.
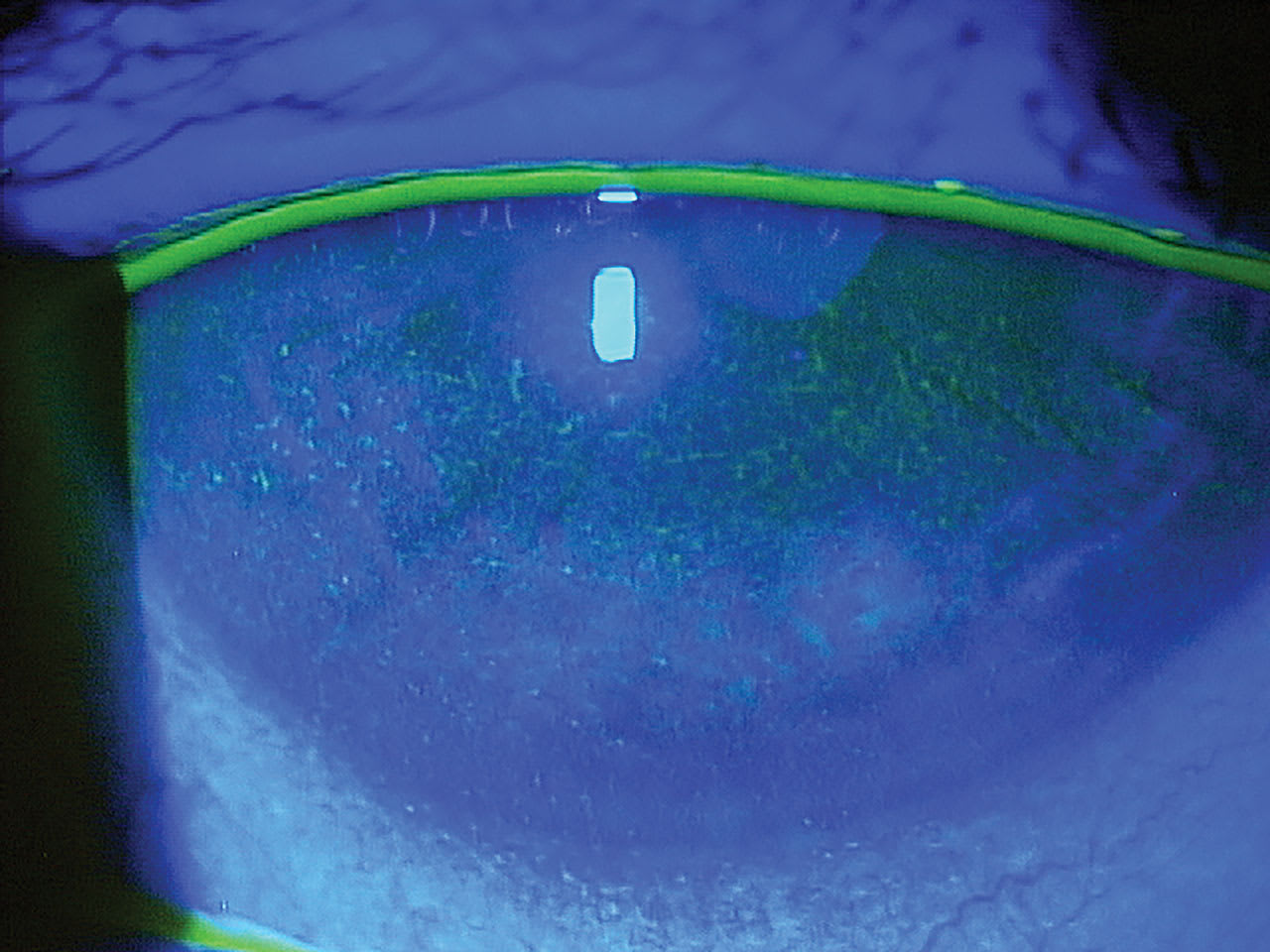
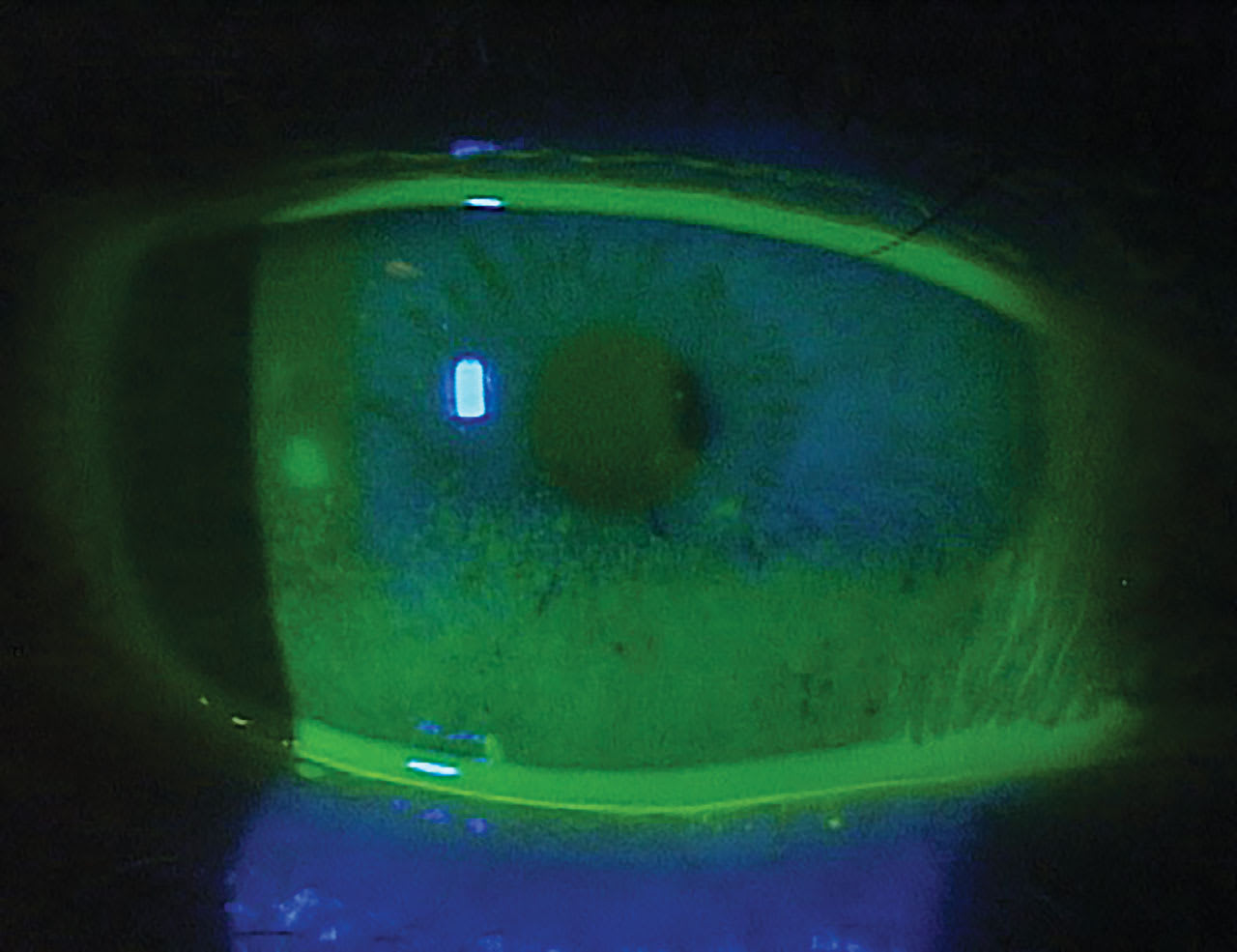
Conduct a full anterior segment workup for corneal epitheliopathies for these patients, including assessing tear volume and stability and blink rate, which is reduced with neurotrophic keratopathy.9
Corneal sensitivity testing is of paramount importance in the diagnosis of neurotrophic keratitis. This can be accomplished qualitatively using a simple cotton wisp or strand of dental floss, or more quantitatively with sophisticated aesthesiometers. Sensitivity testing should be performed centrally and in 4 quadrants across the cornea.5,10 Regardless of instrumentation, this simple yet highly critical test will guide a clinical diagnosis and assess the need for more aggressive therapies specific to neurotrophic disease.
If sensitivity is normal, causes for the clinical signs of epitheliopathy must be considered. If reduced corneal sensitivity is confirmed, an etiology may be determined by obtaining a thorough clinical history. If no neural insult can be recalled or identified, blood workup and/or neuroimaging may be necessary to find the cause.5
Neuropathic pain: Despite significant reports of discomfort and pain, a neuropathic patient may show minimal or no clinical signs.6 Their disease lies not in the deficiency of corneal nerve-driven tissue support but in the hypersensitivity and overaction of corneal nerve signaling. Though this is still poorly understood, it is theorized that damage to sensory nerve fibers causes persistent pain signaling that, over time, leads to lowered stimulatory thresholds and stronger and seemingly exaggerated pain responses (“sensitization”). This damage can initially occur peripherally at the ocular surface or may extend to the trigeminal nuclei or higher structures of the central nervous system.11
These patients may seem overly sensitive to normally non-noxious stimuli like light or ambient air flow. Despite complaints of pain from the patient, the surrounding tissue is generally unaffected. Unlike with neurotrophic disease, the practitioner may not be able to find “proof” of the patient’s pathology when they experience neuropathic pain. Chronic neuropathic pain sufferers of many types, both ocular and non-ocular, often have comorbidities such as depression, anxiety, and posttraumatic stress disorder.6 It is unknown whether these conditions play roles of either cause or effect (or both), but comanagement with a mental health professional can be helpful in many of these cases.12
An important diagnostic test for neuropathic corneal pain is to apply topical corneal anesthesia. If the patient reports significant relief, then it may be concluded that the neural insult is likely peripheral and potentially easier to treat. If only some or no relief is noted, the insult may involve the central nervous system and will be far more challenging to address, as pain symptoms can persist despite treatment of ocular surface inflammation.6
Neural dysfunction: It is not known why damage to nerves can result in anomalous pain signaling, though ocular inflammation and the resulting neuroinflammation has been pointed to as a substantial factor.6,13,14 Additionally, neurotrophic and neuropathic nerve damage do not have to be mutually exclusive; it is possible for a patient to suffer from corneal nerve hypoesthesia and have anomalous pain signaling. A patient like this may report significant chronic pain and yet test positive for reduced corneal sensation.14 A simplified thought process that can be used to differentiate between neural dysfunctions and dry eye can be found in Figure 3, though it does not demonstrate the frequent overlap between these conditions.
Treatment Options
Understanding the differences between neurotrophic and neuropathic disease is important in selecting treatment.5,6These conditions share similarities in early treatment options. The practitioner should start by eliminating identifiable inciting factors. This may involve switching topical drops containing preservatives to their preservative-free formulation or discontinuing the drop entirely. Additional early treatment choices include treating active ocular infections, lid-induced exposure or irritation, and ocular surface dryness. More aggressive options for ocular surface protection include bandage contact lenses, punctal occlusion, and scleral lenses, and they may be necessary treatments for both neurotrophic and neuropathic diseases.
Once the ocular surface is addressed, more specific treatments for each type of neural abnormality may be employed. Bandage contact lenses and scleral lenses offer protection of the ocular surface while allowing for epithelial regeneration and growth. Other nonsurgical options to replenish healing neurotrophic factors include the use of autologous serum tears, platelet-rich plasma drops, and topical human recombinant nerve growth factor. Amniotic membranes may also be used, either surgically through transplantation or topically applied in-office.5,15
The recent approval of a human recombinant nerve growth factor eye drop provides an additional treatment option for patients who have neurotrophic keratitis.16 A multicenter, randomized, double-masked, and vehicle-controlled study of the drug demonstrated significant improvements in corneal healing, including reductions in persistent epithelial defect sizes and disease progression rates, compared to controls, and was well tolerated by patients.17 This drug is readily available for practitioners to prescribe, but patients should be educated on a few important points before use: it requires a commitment to a somewhat grueling regimen of drop preparation and dosing 6 times per day for 8 weeks.16
Additionally, with progressive corneal healing and improved corneal sensation, the previously neurotrophic patient may begin to feel significant discomfort and/or pain.18 Despite this being a positive finding for clinicians, the patient often requires encouragement to continue their course of this medication to completion for best long-term results.
Because neurotrophic keratopathy is relatively rare, studies regarding treatment include small populations, retrospective studies, or case series. A systematic review and meta-analysis of 20 studies found that recombinant nerve growth factor, autologous serum, corneal neurotization, and amniotic membrane transplantation have similar percentages of patients who experience complete corneal healing, although only nerve growth factor and amniotic membrane transplantation showed improved visual acuity after treatment.14 Other studies have explored the use of topical insulin as a potential treatment, with results showing promising results for both diabetic and nondiabetic neurotrophic keratitis as well as other ocular surface diseases.16-18
For neurotrophic keratitis, surgical options include tarsorrhaphy, conjunctival flap coverage, corneal transplant, and corneal neurotization.5 Corneal neurotization is a complex surgery that involves redirecting a healthy nerve or connecting a new nerve via grafting to restore sensation to the cornea.19
More specific treatments for neuropathic pain are similar to those for neurotrophic disease. Scleral lenses not only protect the ocular surface but also limit exposure to the ambient air flow that these patients find intolerable.6Neuroregenerative treatments such as autologous serum, platelet-rich plasma drops, and amniotic membranes may also benefit these patients.
However, in a neuropathic pain patient, direct treatment of inflammation may be more useful.4,6 This can be accomplished via topical steroids, such as loteprednol, and immunomodulating agents such as cyclosporine or lifitegrast. Ideally, topical anti-inflammatory preparations should be preservative free. Other, newer topical options that have shown promise include lacosamide and low-dose naltrexone.4 After months or years of successful scleral lens wear, some patients may become inexplicably intolerant of their lenses, despite a good fit. This adds to the difficulty in treating these patients.21
When neuropathic pain is more central in origin, systemic medication is often required to provide relief. Advocate for these patients and comanage them with neuropathic pain specialists.6 Common medications used for both ocular and nonocular neuropathic pain include tricyclic antidepressants (nortriptyline and amitriptyline), anticonvulsants (carbamazepine, gabapentin, and pregabalin), and opioid agents (naltrexone and tramadol).6,22 Nonmedication treatments may include psychological therapy, lifestyle modifications, acupuncture, and neuromodulation.6,23 Systemic treatment of these patients requires time and patience and is beyond the scope of most primary care optometric practices.
Understanding the similarities and differences between these neurotrophic and neuropathic conditions can help practitioners address neurological corneal pathology in ways that traditional dry eye therapies cannot. Prompt diagnosis of neuropathy in patients who have signs or symptoms of ocular surface disease is key to ensuring that they receive appropriate treatment strategies for their condition.
References
1. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539-574. doi: 10.1016/j.jtos.2017.05.001
2. “Neurotrophic.” Merriam-Webster.com Medical Dictionary, Merriam-Webster.Accessed March 7, 2025. merriam-webster.com/medical/neurotrophic
3. “Neuropathy.” Merriam-Webster.com Dictionary, Merriam-Webster. Accessed March 7, 2025. merriam-webster.com/dictionary/neuropathy
4. Nortey J, Smith D, Seitzman GD, Gonzales JA. Topical therapeutic options in corneal neuropathic pain. Front Pharmacol. 2022;12:769909. doi: 10.3389/fphar.2021.769909
5. Neurotrophic Keratopathy Study Group. Neurotrophic keratopathy: an updated understanding. Ocul Surf. 2023;30:129-138. doi: 10.1016/j.jtos.2023.09.001
6. Dieckmann G, Goyal S, Hamrah P. Neuropathic corneal pain: approaches for management. Ophthalmology. 2017;124(11S):S34-S47. doi: 10.1016/j.ophtha.2017.08.004
7. Soifer M, Gomez-Caraballo M, Venkateswaran N, Jay GW, Perez VL. Associated neurotrophic keratopathy in complex regional pain syndrome. Cornea. 2021;40(12):1600-1603. doi: 10.1097/ICO.0000000000002684
8. Mannis MJ, Holland EJ. Neurotrophic keratitis. In Cornea, 5th Edition. Elsevier. March 5, 2021.
9. Syed SF, Marshall A, Hossain P, Sadiq SA. Blink reflex in neurotrophic keratopathy: an electrophysiological evaluation. Ophthalmic Plast Reconstr Surg. 2022 Sep-Oct 01;38(5):433-437. doi: 10.1097/IOP.0000000000002141
10. Crabtree JR, Tannir S, Tran K, Boente CS, Ali A, Borschel GH. Corneal nerve assessment by aesthesiometry: history, advancements, and future directions.Vision (Basel). 2024;8(2):34. doi: 10.3390/vision8020034
11. Labetoulle M, Baudouin C, Calonge M, et al. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019;97(2):137-145. doi: 10.1111/aos.13844
12. Di Zazzo A, De Gregorio C, Spelta S, Demircan S. Mental burden of ocular surface discomfort. Eur J Ophthalmol. 2024. [Online ahead of print] doi:10.1177/11206721241305661
13. Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology. 2018;129(2):343-366. doi: 10.1097/ALN.0000000000002130
14. Yavuz Saricay L, Bayraktutar BN, Kenyon BM, Hamrah P. Concurrent ocular pain in patients with neurotrophic keratopathy. Ocul Surf. 2021;22:143-151. doi: 10.1016/j.jtos.2021.08.003
15. Roumeau S, Dutheil F, Sapin V, et al. Efficacy of treatments for neurotrophic keratopathy: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2022;260(8):2623-2637. doi: 10.1007/s00417-022-05602-z
16. Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmology. 2020;127(1):14-26. doi: 10.1016/j.ophtha.2019.08.020
17. Balbuena-Pareja A, Bogen CS, Cox SM, Hamrah P. Effect of recombinant human nerve growth factor treatment on corneal nerve regeneration in patients with neurotrophic keratopathy. Front Neurosci. 2023;17:1210179. doi: 10.3389/fnins.2023.1210179
18. Soares RJDSM, Arêde C, Sousa Neves F, da Silva Fernandes J, Cunha Ferreira C, Sequeira J. Topical insulin-utility and results in refractory neurotrophic keratopathy in stages 2 and 3. Cornea. 2022;41(8):990-994. doi: 10.1097/ICO.0000000000002858
19. Almeida J, Costa TR, Vivas M, et al. Long-term results of topical insulin treatment for persistent corneal epithelial defects. J Ophthalmic Vis Res. 2024;19(4):397-404. doi: 10.18502/jovr.v19i4.13977
20. Krolo I, Behaegel J, Termote K, et al. The role of topical insulin in ocular surface restoration: a review. Surv Ophthalmol. 2024;69(5):805-817. doi: 10.1016/j.survophthal.2024.04.003
21. Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100(1):128-34. doi: 10.1136/bjophthalmol-2014-306280
22. Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 2015;90(4):532-545. doi: 10.1016/j.mayocp.2015.01.018
23. Attal N, Bouhassira D. Advances in the treatment of neuropathic pain. Curr Opin Neurol. 2021;34(5):631-637. doi: 10.1097/WCO.0000000000000980




